Adaptive 2
Study-Specific Pre-Screen Form
The main purpose of this study is to see if smokers who respond early to alternative nicotine products can successfully maintain their smoking reduction by continues use of these noncombustible nicotine alternatives for up to 6 months. These alternative nicotine products include nicotine gum, nicotine lozenges, nicotine patches, oral nicotine pouches, or e-cigarettes.
Where can I find more information on this study?
Details on this study are available on our website at https://roseresearchcenter.com/current-studies/
What are the criteria to participate?
- Able to read, understand, and willing to sign an informed consent form (ICF) and complete questionnaires written in English
- Between the ages of 22-65
- Currently smoke 10 or more cigarettes per day
- No current serious medical conditions
- Not pregnant, breastfeeding, or planning to become pregnant
What is the purpose of the study?
The purpose of this study is to see if smokers who respond early to alternative nicotine products can successfully maintain their smoking reduction by continues use of these noncombustible nicotine alternatives for up to 6 months. These alternative nicotine products include nicotine gum, nicotine lozenges, nicotine patches, oral nicotine pouches, or e-cigarettes.
After an extensive review of the scientific evidence regarding the NJOY ACE e-cigarette offered to you in this study, The Food and Drug Administration (FDA), has concluded that complete switching to them is less harmful than smoking conventional cigarettes and authorized their marketing as being “appropriate for the protection of public health.”
The nicotine patches, gum and lozenges being offered to you have been determined by the FDA to be safe and effective products to aid smoking cessation. The nicotine pouches offered to you in this study are legally marketed products currently being reviewed by the FDA for their health effects and effect on public health.
How long is the study?
Early nicotine responders will be randomized to continued use of alternative nicotine products for either 12 weeks (total time) or 24 weeks (total time). All early responders will be contacted at 9-months, to evaluate their current smoking status. Study duration for early nicotine non-responding participants will be approximately 3-4 weeks (at the end of Telehealth 3).
Will I be compensated for my participation in this study?
You will be compensated for your time and inconvenience related to your participation in this study via PayPal. You will need to create a PayPal account to receive payment for participation in this study. If you do not complete the study, for any reason, you will be paid for the portions of the study you do complete according to the following schedule:
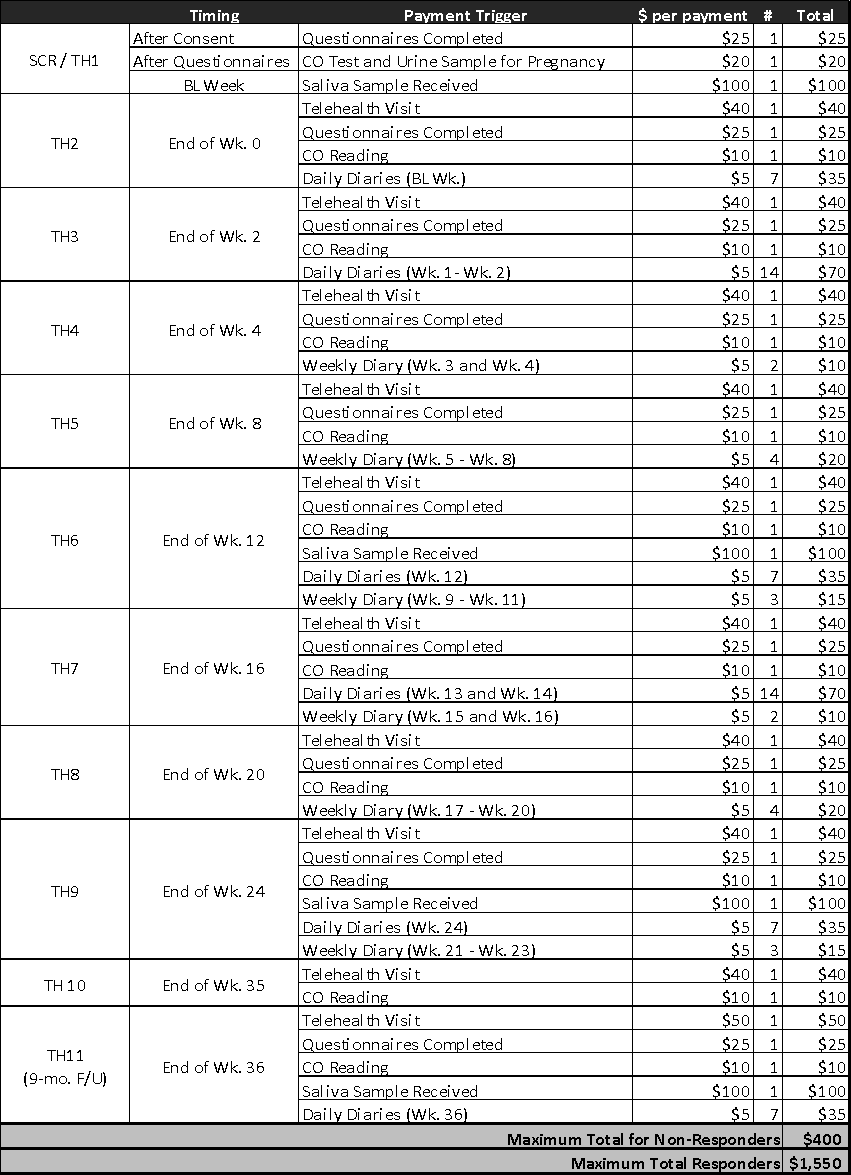
Payment received as compensation for participation in research is considered taxable income. You are responsible for paying any state, federal or Social Security taxes on the money you receive. If your total payment exceeds $600 in any one calendar year, we are required to report this information to the Internal Revenue Service (IRS). You will be required to provide your Social Security number or tax identification number for payment.
Who will have access to my name and the information collected during participation?
Your records will be kept as private as possible under law. Your Information will be stored in limited-access databases. The study staff will have access to these databases. Each of these individuals will be obligated to protect the confidentiality of your Information and to use and disclose it only as described in this document.
Rose Research Center, the Study Sponsor, the FDA, other health authorities, and the IRB may inspect your hard-copy and electronically stored research records which may include your name, address and other personal information that identifies you. If necessary, some of these records may be copied during these inspections.
The results of this research study may be presented at meetings or in publications. However, you will not be personally identified in any presentations or publications.
Further information about the confidentiality and protection of your personal information and protected health information are explained in the “Authorization to Use and Disclose Protected Health Information” Section at the end of this document.
Who is sponsoring the study?
The study is being funded by a grant from Global Action to End Smoking, formerly the Foundation for a Smoke-Free World. Global Action to End Smoking is an independent non-profit organization. The study investigator and study staff are being paid to conduct this study.
What is informed consent?
The informed consent process provides you with the information required to ensure you make an informed decision about participation in the clinical research study. This process will:
- Help you to understand the study
- Give you an opportunity to ask questions and to consider whether to participate
- Obtain your voluntary agreement to participate
- Continue to provide information as the clinical research study progresses
Before participating, we will take you through an Informed Consent Form (ICF) that outlines important information to consider before joining the study. The study staff will review this document with you and answer any questions you may have. This document provides details of the study, such as study expectations, duration, and what you will need to do. All known possible risks and potential benefits are also explained in the ICF. After you have read and discussed all the information about the study, you can decide whether or not to sign the consent form. This consent form is not a contract, and you are always free to leave the study at any time and for any reason, with no penalty to you. Additionally, during your participation, the study staff will let you know in a timely manner of any information that may change your mind about participating.
Other Questions
If you have any other questions, please call us at 1-866-984-7673.